Cancer care in Spain
Life expectancy in Spain in 2020 was almost two years higher than the EU average, despite a fall of 1.6 years because of the high mortality registered during the COVID-19 pandemic. Before the pandemic, life expectancy had increased steadily in Spain by more than four years between 2000 and 2019, reaching 84 years in 20191 .
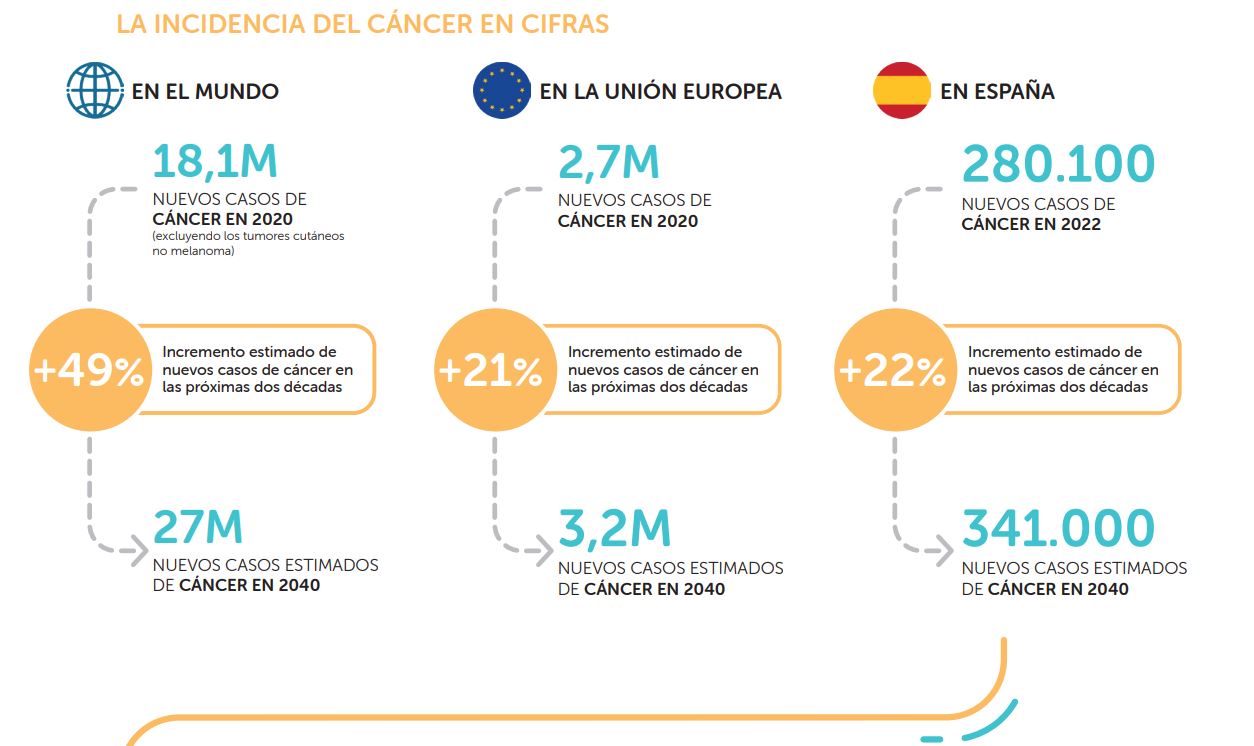
Although the pandemic severely disrupted cancer screening and diagnosis in the country, in 2022 around 276,000 new cases of cancer are expected to be diagnosed in Spain, according to the Spanish Network of Cancer Registries (REDECAN), a figure that the Spanish Society of Medical Oncology (SEOM) estimates will increase to 341,000 new cases by 2040. In addition, SEOM estimates that cancer mortality is estimated to increase from 113,000 cases in 2020 to more than 160,000 in 20402. Between 2010 and 2018, according to AECC (Spanish Association Against Cancer) data and the Burden of Cancer In Spain report by Omakase Consulting, five-year cancer prevalence in Spain increased by 0.24% yearly on average, from 1,225 cases to 1,246 cases per 100,000 inhabitants; incidence increased by 1.45%, from 444 cases to 491 per 100,000 inhabitants; and mortality increased by 1.57%, from 211 cases to 235 per 100,000 inhabitants.3,4
In words of professor Eduardo Díaz-Rubio, one of the most prominent authorities in Oncology in Spain and President of the Royal National Academy of Medicine (founding member of All.Can Spain), “cancer in Spain is currently a major challenge due to its high incidence, mortality and prevalence. Today, it has become the first cause of death in men and the second in women, being responsible for the 27% of the deaths. The forecasts are also negative, as the incidence of cancer will increase in the coming years. In addition, cancer generates a high burden of temporary and permanent disability, estimating total annual costs of more than 7,000 million €, which represents 10% of Spanish health expenditure or what is the same 0.66% of GDP.
The good news is that 58% of cases present survival of more than 5 years, although this varies according to the type of tumor. However, this survival could be clearly improved, according to the data available in the countries that present the best figures. The challenges in cancer go through optimizing a better organization and management; a greater support for basic research; a systematized implementation of translational research in hospitals; strong support for clinical trial policy; and better access to the newest therapies as well as the biomarkers that make precision medicine possible.
Of course it is necessary to guarantee that all Spanish people have the same possibilities as regards screening, diagnosis and treatment. The focus should be not only for equity, but also for quality and patient satisfaction. To achieve all this, it is necessary to measure and evaluate so that a comparative and competitive medicine were a reality. The excellence in results is the main objective. Finally an adequate financing is needed with a finalist state budget, and that allows a medicine in cancer sustainable”5.
In conclusion, with increasing life expectancy, prevalence of cancer is expected to continue growing. At the same time, scientific advances to diagnose and treat the condition are also increasing, and we need to ensure cancer patients have equal access to the latest scientific advances, including biomarkers and new treatments. The issue of limited budgets and the affordability of scientific advances is becoming a challenge. Health and long-term care expenditure in Spain are also expected to rise in future as a share of GDP due to population ageing and technological progress. One way to get more resources is to look at current inefficiencies in cancer care that do not add value to increase patient outcomes. The future of oncology seems to be promising; however, the current approach to oncological care in Spain may not be ready for tomorrow. Further efficiency gains in health and long-term care delivery will be needed to address the growing needs of an ageing population in an affordable way.
All.Can Spain initiative
All.Can Spain has launched an ‘open’ platform, in collaboration with Bristol-Myers Squibb and Amgen, bringing together some of the leading cancer-related insitutions and patients associacions in Spain. The platform has four main objectives, which will be achieved through the activity of its Scientific Committee:
- Contribute to the generation and dissemination of knowledge on the burden of cancer in Spain and ways for optimizing cancer care in the country, putting the patient at the centre
- Capillarize the knowledge generated to main stakeholders and key decision makers for moving into action
- Propose multi-channel actions to bring forward solutions for the inefficiencies identified to increase patient health outcomes
- Position cancer as a priority in health policy in Spain
The initiative is supported currently by leading organisations including cancer patient associations, scientific societies, national council of Pharmacists and Nursery, and pharmaceutical companies. To this day, its Institutional Members are:

Activities and milestones
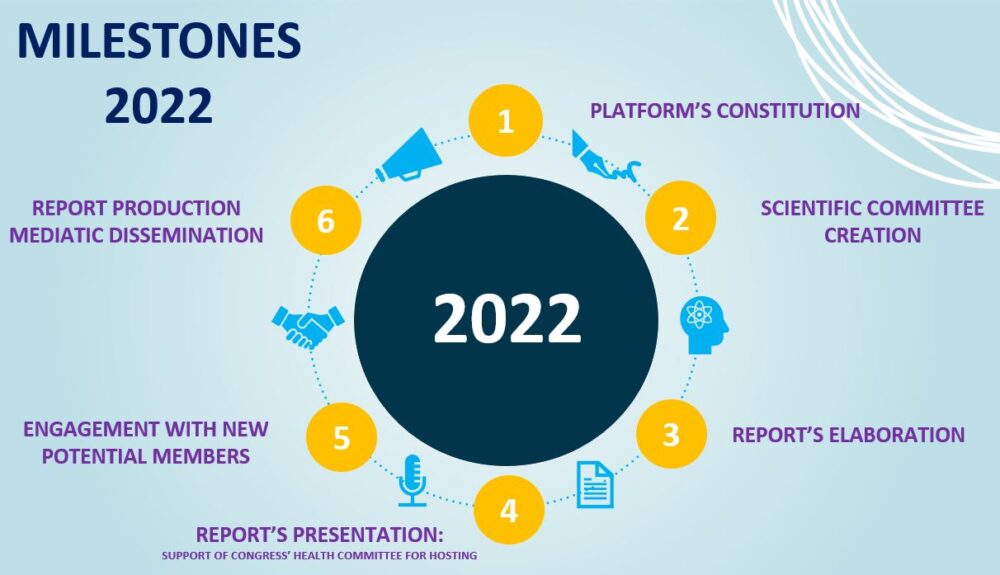
2022
All.Can Spain´s Scientific Committee had already been set up (see ‘Members’ tab) and produced its 1st report in 2022, its 1st contribution to substantially improve the cancer patient experience within Spanish Health System through capillarization and alliances with key decision-makers in the country. The Committee, formed by some of the leading institutions relating cancer in Spain, has identified a series of recommendations centred on the optimization of the patient´s experience up until diagnosis, inspired by the one produced by All.Can Canada Optimizing Diagnosis in Canadian Cancer Care.
You can find All.Can Spain´s full report here, and the executive summary here (Spanish) (English).
A summary of the All.Can Spain initiative in 2022 is shown below:
References
- OECD/European Observatory on Health Systems and Policies (2021), Spain: Country Health Profile 2021, State of Health in the EU, OECD Publishing, Paris/European Observatory on Health Systems and Policies, Brussels. Available here: https://health.ec.europa.eu/system/files/2021-12/2021_chp_es_english.pdf [accessed: August 2018]
- Las Cifras del Cáncer en España 2022, SEOM, febrero 2022 Available here: https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf
- La carga del cáncer en España Available here: http://www.omakaseconsulting.com/wp-content/uploads/2018/04/omakase-lab-3-2018--burden-of-cancer-in-spain.pdf [accessed: August 2018]
- Observatorio del Cáncer AECC Available here: http://observatorio.aecc.es/ [accessed: August 2018]
- Cancer in Spain: situation in 2019. Anales RANM Año 2019 · número 136 (01) · páginas 25 a 33 Available here: https://analesranm.es/wp-content/uploads/2019/numero_136_01/pdfs/ar136-01-rev06.pdf
Scientific Committee - Patients
Bernard Gaspar
President of the Spanish Association of People Affected by Lung Cancer (AeACAP)
Roberto Saldaña
Director of Innovation and Citizen Participation; EUPATI Spain
Enric Barba
Patient Advocate of the Melanoma Spain Association
Scientific Committee - Scientific societies and institutions
Dr. Luis Paz-Ares
President of the Spanish Association for Cancer Research (ASEICA)
Antonio Blanes
Director of Technical Services of the General Council of Official Associations of Pharmacists (CGCOF)
Guadalupe Fontán
Director of the Research Institute of the General Nursing Council (CGE)
Dr. Rafael López
President of the ECO Foundation for Excellence and Quality in Oncology
Dr. Mariano Provencio
Member of the Royal National Academy of Medicine (RANM); Head of the Oncology Department of the Puerta de Hierro University Hospital
Dra. Candela Calle
Board of Directors of the Spanish Society of Health Managers (SEDISA)
Technical Secretariat
Pepe Fernández-Rúa
Managing Partner at Cariotipo
Diego Suárez
Senior Consultant at Cariotipo
All.Can Spain conveys the urgency of accelerating cancer diagnosis to the Health spokespersons of the Congress of Deputies
All. Can Spain publishes its 1st Report, focused on improving the patient´s cancer care experience up until diagnosis
All. Can Spain’s Scientific Committee creation and 1st meeting
All. Can Spain Constitution Ceremony: The international platform All. Can is consolidated in Spain to contribute to achieving improvements in the care policies for cancer patients
1st All.Can Informative Meeting in Spain
All.Can Spain reveals results of working group sessions
The All.Can Spain initiative has revealed the results of its first two working group sessions.
All.Can Spain initiative presented at 2nd Congress of Patient Organisations
The new All.Can Spain initiative has officially launched, with a presentation at the II Congreso de Organizaciones de Pacientes.
All.Can Spain´s Scientific Committee (2022 onwards)
- FULL REPORT: Download the full report The cancer patient experience: optimising the circuit and improving care and coordination until diagnosis. Eight recommendations for a real change (Spanish) (December 2022)
- EXECUTIVE SUMMARY: Download the executive summary The cancer patient experience: optimising the circuit and improving care and coordination until diagnosis. Eight recommendations for a real change (Spanish) (English) (December 2022)
Working group session results
All.Can Spain poster
Download the poster presented at II Congreso de Organizaciones de Pacientes on 8–9 October 2018: The initiative All.Can Spain to detect inefficiencies in the management of cancer, improve health outcomes and empower cancer patients in Spain
Disclaimer:
The All.Can initiative is made up of leading representatives of patient organisations, policymakers, healthcare professionals, science and industry. All the publications produced under the initiative reflect the consensus of All.Can members who have full editorial control. The All.Can initiative in Spain is funded by Bristol Myers Squibb and Amgen. None of the content developed in discussions and activities of the All.Can initiative contains direct or indirect references to specific medicinal products or therapies. Use of content from this website is permitted provided that the source of information is clearly mentioned.